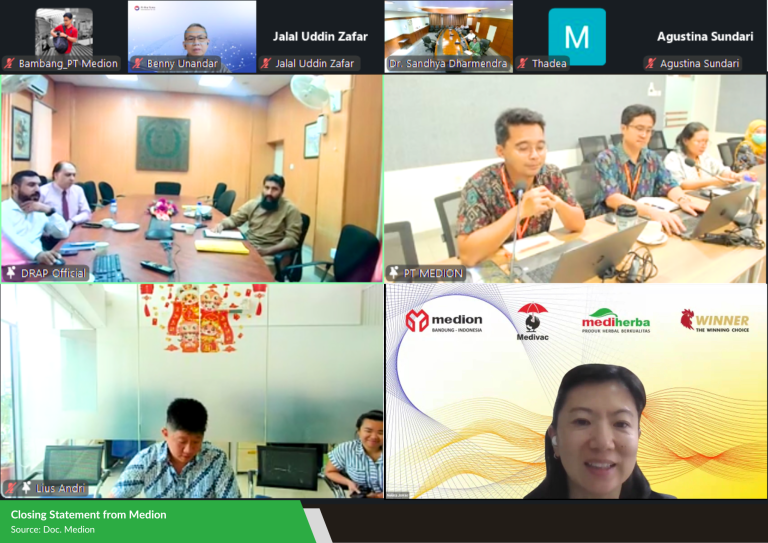


On September 4, 2025, Medion underwent another virtual Good Manufacturing Practice (GMP) inspection by Pakistan’s Drug Regulatory Authority (DRAP) for its inactivated vaccine production facility. This inspection is part of the process to renew registrations for Medion’s existing products and register new ones in Pakistan, which requires passing a GMP inspection by the regulatory authorities.
The inspection team included Dr. Muhammad Zubair Masood (Deputy Director of BE&R) and Dr. Muhammad Ashfaq (Deputy Director of MDMC) from DRAP, alongside Medion’s distributor team in Pakistan, export agents from AVI (Arthavena International Pte Ltd.), and Medion’s internal team. The event began with a presentation about Medion’s company profile and its biological products, followed by a virtual tour of the production facilities—from raw material preparation and production equipment to vaccine manufacturing, quality control, packaging, storage, and laboratory and animal testing areas.
The inspectors were very pleased with how smoothly the inspection went and how efficiently it was completed. They found Medion’s vaccine production facilities to be well-organized, clean, and in line with international standards. They also praised the Medion team for providing clear, concise, and well-structured explanations that helped the inspection proceed without any issues. Previously, DRAP certified Medion’s active vaccine production facility in 2024. Successfully passing the inspection for the inactivated vaccine facility opens up greater opportunities for Medion to expand its vaccine exports to Pakistan, support poultry health in the region, and strengthen its presence in the global veterinary health market.
